What's New?
The 2001-2002 Beckman Scholars: Nicholas Ryan Conley, PhD
Beckman research project in the Willson group: Nonlinear optics: Designing new materials |
Introduction
The field of nonlinear optics (NLO) is based on the study of effects and phenomena related to the interaction of intense coherent light radiation with matter. Under such conditions, materials react nonlinearly to the electric field of light incident upon them, resulting in the production of new frequencies of light. In second-harmonic generation, light of angular frequency w is converted to light of angular frequency 2w. Materials with high second-order nonlinear optical coefficients serve as catalysts for this process, possessing vast potential for use in a variety of photonic systems, such as high speed optical modulators, ultrafast optical switches, and high density optical data storage media.
Although inorganic materials are most commonly used for NLO applications, organic polymers have the potential to surpass the performance of current inorganic materials due to their high electro-optic coefficients, fast response time, lower dielectric constants, and flexibility of fabrication methods. Poled organic polymers capable of efficient second-harmonic generation have been reported and have several structural features in common: they are non-centrosymmetric, have an extended pi system, and contain both electron-donor and electron-acceptor groups. Unfortunately, most polymers exhibit temporal instability at high temperatures, eventually undergoing dipole reorientation. The design and synthesis of thermally stable, organic NLO polymers is therefore a worthy goal.
Experimental
The reaction scheme is shown below:
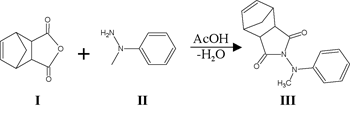
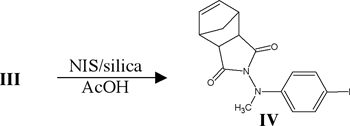
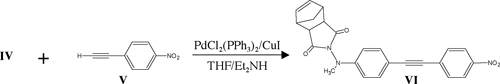
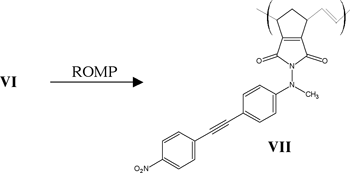
Results and Discussion
As of this writing, we have isolated and characterized all intermediates leading up to and including VI. Substantial literature precedent exists for the polymerization of VI, and therefore, my research project should culminate in the synthesis of VII shortly.
It is interesting to note that in the course of this work, we discovered that a structure previously reported in the literature to be N-aminomaleimide VIII was actually aminoisomaleimide IX.
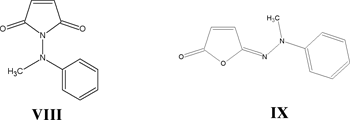
Conclusion
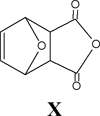
We have determined that the structures previously reported to be N-aminomaleimides are actually aminoisomaleimides and we have developed the first general preparation of authentic N-aminomaleimides by the condensation of the maleic anhydride/cyclopentadiene adduct I or the maleic anhydride/furan adduct X with a hydrazine derivative. If the latter is chosen, the retro Diels-Alder reaction can be effected with heating to afford compounds similar to VIII. These routes enable the preparation of valuable, yet previously unobtainable intermediates in the synthetic route to the key monomers that will be used to prepare new, thermally stable polymers with high second order nonlinear optical coefficients.
Created and maintained by Ruth Shear. Comments to author at DrRuth@mail.utexas.edu
Created Thu Mar 7th 2002. Last modified Mon, Mar 16, 2015.
