What's New?
The 2012-2013 Beckman Scholars: Ricky Garza
| Faculty Mentor: Professor Stephen F. Martin Length of term: Summer 12, Fall 12, Spring 13, Summer 13 Honors & Awards:University Honors (Fall 09, Spring 10, Fall 10, Spring 11, Fall 11, Spring 12, Fall 12); Emory and Ella Peterson Unrestricted Endowed Presidential Scholarship (2012); Aspire Professor's Choice Award (2012); Proctor & Gamble award for excellence in chemical research (2012); College of Natural Sciences Summer Fellowship (2011); College of Natural Sciences Book Award (2010). Publications: Where is he now? Graduated with Bachelor of Science (Chemistry) with Honors, with Special Departmental Honors in Chemistry, May 2013. Ricky is currently at dental school at Harvard University. How can I contact him? rickygarzajr at gmail.com |
 |
Beckman research project in the Martin group
Total synthesis of Jiadifenolide
The research group's focus is to synthesize natural and unnatural products that are of biological or structural interest. To this end, novel synthetic strategies are also explored that can be used to design complex targets.Specifically, this research project is focused on neurotrophins, a family of proteins that have shown to regulate nervous system growth and mediate neuronal survival.1 This characteristic has drawn much interest from the medical community in the hope that they may be used to treat acute nervous system injury or chronic neurodegenerative diseases (i.e. Alzheimer's disease). However, due to the inability of these proteins to persist in the body for an extended period and cross the brain-blood barrier, molecules that mimic the effects of neurotrophins, or can at least induce biosynthesis of these neurotrophic factors, have received much interest. Fukuyama and co-workers isolated three sesquiterpenoids, (-)-jiadifenolide (1) and jiadifenoxolanes A (2) and B (3) from the pericarps of Illicium jiadifengpi (Figure 1).2
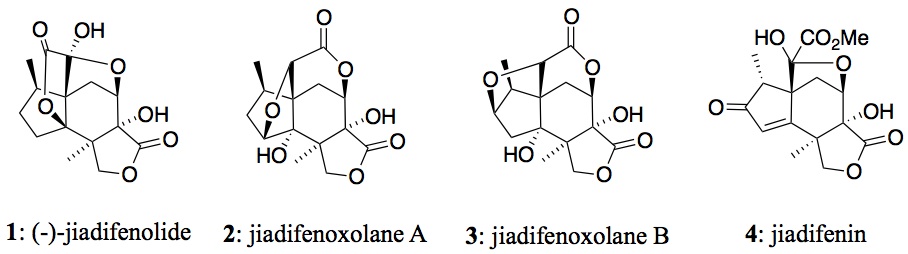
Of these compounds, 1 and 2 have shown potent activity in promoting neurite growth when added to primary cultures containing rat cortical neurons (10 nM and 1 microM, respectively). The objective is to synthesize Jiadifenolide in a more expedient and concise manner than previously reported.3 Moreover, the key step of the proposed synthesis is an intramolecular cyclization mediated by samarium(II) iodide, a compound that has been recognized as a premier reagent in synthetic chemistry due to its potent combination of reactivity and selectivity (Figure 2).4
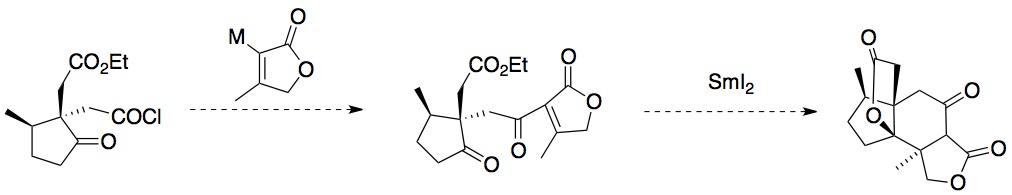
Successful completion of the proposed route could lead to the use of this synthetic approach to synthesize the family of related natural products, molecules that also been shown to have similar promising medicinal behavior.
1. Hefti, F. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 239-267.
2. Kubo, M.; Okada, C.; Huang, J.-M.; Harada, K.; Hioki, H.; Fukuyama, Y. Org. Lett. 2009, 11, 5190-5193.
3. Xu, J.; Trzoss, L.; Chang, W. K.; Theodorakis, E. A. Angew. Chem. Int. Ed. 2011, 50, 3672-3676.
4. Nicolaou, K. C.; Ellery, S. P.; Chen, J. S. Angew. Chem. Int. Ed. 2009, 48, 7140-7165.
Created and maintained by Ruth Shear. Comments to author at DrRuth@mail.utexas.edu
Created Wed Jun 6th 2007. Last modified Mon, Mar 10, 2014.