What's New?
The 2009-2010 Beckman Scholars: Travis Turner
| Faculty Mentor: Professor Stephen F. Martin Length of term: Summer 09, Fall 09, Spring 10, Summer 10 Honors & Awards:University Honors (Fall 05, Spring 06, Fall 06, Spring 07, Spring 08, Spring 09); Unrestricted Endowed Presidential Scholarship (2007); Louis Weisberg Memorial Chemistry Scholarship (2009) Publications:Hoshino, Kazunori; Turner, Travis C.; Kim, Sunmin; Gopal, Ashwini; Zhang, Xiaojing, ÒSingle Molecular Stamping of a Sub-10-nm Colloidal Quantum Dot ArrayÓ Langmuir (2008), 24(23),13804-13808. Where is he now? Graduated with Bachelor of Science in Chemistry with Special Departmental Honors in Chemistry, May 2010. Travis completed an MS at Scripps Research Institute, and is currently at graduate school in the Department of Materials Science and Engineering at UT Austin. How can I contact him? tcturner at scripps.edu |
 |
Beckman research project in the Martin group:
Download a copy of Travis' Research report, entitled Concise syntheses of (±)-roelactamine and structural analogs
Multicomponent reactions (MCRs) provide a facile means for the preparation of diverse chemical structures that can be elaborated into both natural product libraries and druglike molecules.1,2 Recently, our laboratories have developed a novel MCR involving the sequential reaction of a primary amine, an aldehyde, an acid chloride, and a nucleophile to produce a general intermediate which is then subjected to cyclizations and functional group transformations to afford a highly-functionalized heterocyclic scaffold.2-4 One advantage of our MCR is the commercial availability of a wide variety of starting materials, allowing diverse structures to be created in a small number of steps. To illustrate our MCR in natural product synthesis, we have recently completed the first total synthesis of the isopavine alkaloid (±)-roelactamine in just four steps from commercially available starting materials (Scheme 1).3 The acid chloride B was prepared from commercially available glyoxylic acid in one step. Roelactamine is the only known naturally occurring isopavine alkaloid with a lactam moiety.
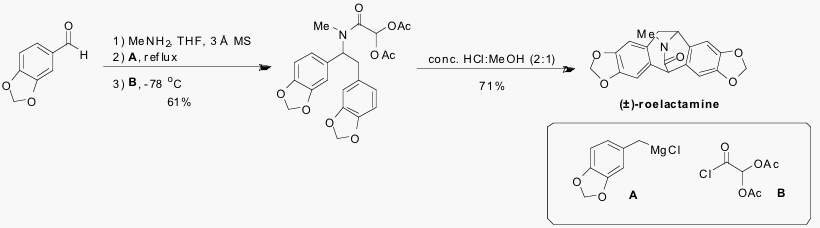
The primary goal of the proposed research is to shorten the synthesis of (±)-roelactamine to just three steps by using commercially available dichloroacetyl chloride (C) (Scheme 2).
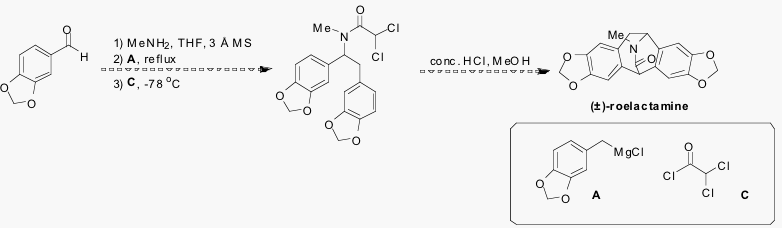
If successful, this synthesis may provide an even shorter route to (±)-roelactamine. In addition, this synthesis would provide a means to quickly generate a library of roelactamine synthetic analogs for biological activity testing. Synthetic isopavine alkaloids have been shown to act as potent NMDA receptor antagonists.5 Since overstimulation of the NMDA receptor by glutamate has been shown to result in neuronal degeneration in several diseased states and after prolonged oxygen deprivation, a selective NMDA receptor antagonist may provide a neuroprotective effect. Depending on the timeline of this study, other areas involving the use of our MCR may also be explored over the next fifteen months.
1 Ulaczyk-Lesanko, A.; Hall, D. G. Curr. Opin. Chem. Bio. 2005, 9, 266-276.
2 Sunderhaus, J. D.; Martin, S. F. Chem. Eur. J. 2009, 15, 1300-1308.
3 Sunderhaus, J. D.; Dockendorff, C.; Martin, S. F. Org. Lett. 2007, 9, 4223-4226.
4 Sunderhaus, J. D.; Dockendorff, C.; Martin, S. F. Tetrahedron. 2009, doi: 10.1016/j.tet.2009.05.009 (in press).
5 Gee, K. R.; Barmettler, P.; Rhodes, M. R.; McBurney, R. N.; Reddy, N. L.; Hu, L.-Y.; Cotter, R. E.; Hamilton, P. N.; Weber, E.; Keana, J. F. W. J. Med. Chem. 1993, 36, 1938-1946.
Created and maintained by Ruth Shear. Comments to author at DrRuth@mail.utexas.edu
Created Wed Jun 6th 2007. Last modified Mon, Mar 10, 2014.