What's New?
The 2001-2002 Beckman Scholars: William Russell Renthal, MD/PhD
Beckman research project in the Iverson group: Improving antimicrobial cationic peptides |
Summary
I set out to identify the mechanism by which a novel antibiotic kills bacteria and to assess its danger to mammalian red blood cells. I first synthesized and purified the antibiotic compound. I then studied its mechanism with a fluorescent assay which suggests the compound kills bacteria by disrupting their membranes. I am within about a month of supporting these results with studies on synthetic membranes. I have also demonstrated that the antibiotic has essentially no activity against mammalian red blood cells, which highlights its potential for pharmaceutical use. In the next month, I should have enough data to put together a paper discussing the mechanism of action of our novel antibiotic. I am also pursing an interesting effect I noticed about our antibiotic that could have far-reaching implications for drug design in general, so over the next year it is possible that my Beckman project will yield another publication on this exciting observation.
Background
Antibiotic resistance has led to a crisis in the simple treatment of bacterial infections. Thus, there is an urgent need for the development of novel antibiotics with bactericidal activity against resistant strains. Recently, there has been significant interest in the use of natural antimicrobial cationic peptides found in organisms ranging from insects to humans as novel antibiotics. Cationic peptides are naturally produced in specialized tissues to serve as a first line of defense against harmful bacterial infections (reviewed in Zasloff, 2002). The antimicrobial properties of these cationic peptides are apparently due to their tight association with, and thus disruption of, the negatively charged bacterial membrane (figure 1). Because bacterial cell membranes are typically far more negatively charged than mammalian membranes, cationic antimicrobial peptides have tremendous potential as novel human antibiotics. Moreover, since these peptides attack the physical properties of the cell membrane as opposed to specific amino acid residues, quick antibacterial resistance is unlikely. Indeed, these peptides have remained nearly resistant-proof against bacteria for millions of years.
 |
| Figure 1: Proposed mechanism of antimicrobial cationic peptide action |
Miller et al. from the Iverson laboratories here at the University of Texas at Austin have recently developed a class of 1,4,5,8-Naphthalene-tetracarboxylic Diimide-Peptide (NDI) conjugates which have potent antibacterial activity (Miller et al., 2001). The antimicrobial conjugate contained a poly-lysine cationic peptide attached to the NDI backbone, however the antimicrobial activity of the conjugate is far greater than that of the poly-lysine cationic peptide alone. However, it is not clear how this antimicrobial activity occurs or how safe the compound is against mammalian red blood cells.
Results
Synthesis of NDI-Lys7 conjugate
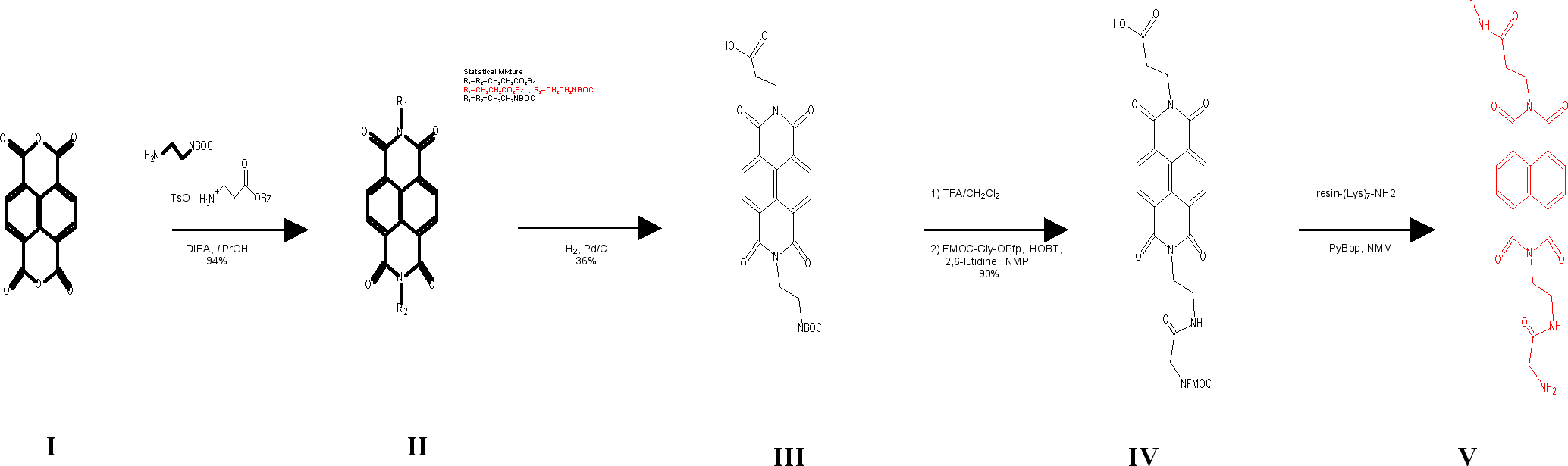
Synthesis of Gly-NDI-peptide conjugate. 1,4,5,8-Naphthalenetetracarboxylic dianhydride I is combined with mono-tert-butoxycarbonylaminoethylamine and _-alanine benzyl ester p-toluene sulfonate salt in 2-propanol. N,N-Diisopropyl-ethylamine is added and heated under reflux to yield a statistical mixture of diimide products II. The mixture is hydrogenated and then separated by a silica column. Fmoc-protected glycine Pfp-ester is combined with III to yield IV, which can then be coupled via SPPS to any peptide on a resin to yield the final product V. Compound V is then purified by FPLC and purity is confirmed by HPLC/ESI mass spectrometry.
Antibacterial activity
Figure 2 shows the activity of the NDI-Lys7 conjugate against B. subtilis bacteria, and indicates that its minimum inhibitory concentration (MIC) is near that of streptomycin.
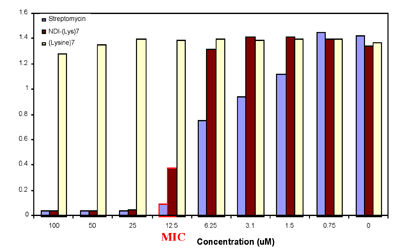
Figure 2. Serial dilutions of NDI-(Lys)7, (Lys)7, and streptomycin are made starting at 100mM in MH media in 96-well microtiter plates. Bacillus Subtilis were diluted to an OD600= 0.1 and 10mL were added to each well. The plates were incubated at 37oC for 18-20 hours. The minimum inhibitory concentrations (MIC) of each compound is considered the lowest concentration required to inhibit growth >=50% of the control.
Mechanism of action
Using a fluorescent dye that monitors the membrane potential of bacteria, we could establish what effect the NDI-Lys7 had on the bacterial membrane potential. In Figure 3 it can easily been seen that at NDI-Lys7 concentrations equal to the MIC, the bacterial membranes are depolarized rapidly.
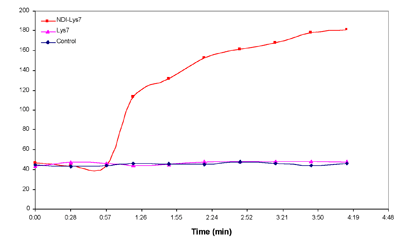
Figure 3. Membrane depolarization assay. a) Mid-log phase cell prior to addition of the cyanine dye. b) The cell membrane after 30 minutes of equilibration in 1 uM dye. c) 12.5uM of NDI-(Lys)7 or (Lys)7 is added and the dye monitors the membrane potential as a function of fluorescence intensity. Higher intensities correlate with depolarization of the membrane. d) Structure of the cyanine dye, 3,3'-Dipropylthiadicarbocyanine iodide (DiSC3(5)). The dye excites at 622nm and emits at 670nm.
Danger to red blood cells
To determine the danger of our compound to red blood cells (RBC) we performed a hemolysis assay as described in Figure 4. Our data suggests that there is no significant danger to RBCs from exposure to our compound against.
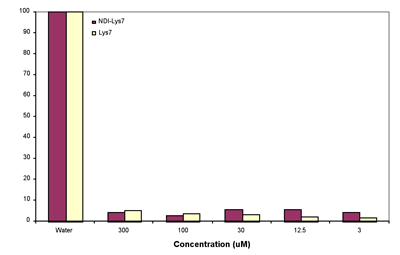
Figure 4. Sheep red blood cells were washed in iso-tonic PBS then diluted to 20% v/v. Cells were incubated with above conc. of NDI-(Lys)7 and (Lys)7 for 1hr at 37oC. Cells were then centrifuged and the Abs416 was read of the supernatant. Abs416 of pure water is considered 100% hemolysis. Hemoglobin absorbs at 416 nm, so we were able to quantify the amount which leaked out of red blood cells upon exposure to our compound.
Conclusions
The NDI-Lys7 conjugate displays antimicrobial activity near that of Streptomycin. Due to the rapid depolarization of bacterial membranes upon exposure to our compound, it suggests that the mechanism by which this activity occurs is related to membrane action. This hypothesis will be further supported with liposome studies shortly. This compound shows great promise for pharmaceutical use since we have demonstrated that shows little to no activity against mammalian red blood cells. If we can optimize its potency just over ten-fold into the nanomolar MIC range, this compound could be of significant interest in fighter human bacterial infections.
Future Directions
We are currently preparing synthetic lipid studies to determine precisely how well our compound interferes with lipid bilayers. With these liposomes we can then quantify the activity against membranes and optimize the potency of our molecule. If we can achieve a nanomolar MIC, our compound could be of great use amidst the current crisis in antibiotic resistant bacteria.
References
Miller CT, Weragoda R, Izbicka E, Iverson BL,."The Synthesis and Screening of 1,4,5,6-Napthalenetetracarboxylic Diimide-Peptide Conjugates with Antibacterial Activity", Bioorganic & Medicinal Chemistry 2001, 9, 2015-2024.Zasloff, M., "Antimicrobial peptides of multicellular organisms," Nature 2002, 215, 389-395.
Created and maintained by Ruth Shear. Comments to author at DrRuth@mail.utexas.edu
Created Thu Mar 7th 2002. Last modified Mon, Mar 16, 2015.